Engineers develop a quick urine test using CRISPR, barcoded DNA and nanoparticles to diagnose for cancer.
Research Paper
Liangliang Hao et al., CRISPR-Cas-amplified urinary biomarkers for multiplexed and portable cancer diagnostics. Nature Nanotechnology. (2023).Engineers from the Massachusetts Institute of Technology (MIT) have developed a new nanoparticle sensor that could enable early diagnosis of cancer with a simple urine test. The sensors can detect different cancerous proteins. They could also be used to distinguish the type of tumour or how it is responding to treatment.
The nanoparticles are designed such that when they encounter a tumour, they shed short sequences of DNA that are eventually excreted into urine. These short DNA sequences act as barcodes containing details about the tumour. By analysing these DNA barcodes, details of a patient’s tumour can be revealed.
An outline of the process is:
- Nanoparticles containing specific DNA barcodes are administered into the patient.
- Cancer associated enzymes cleave these barcodes predictably.
- These cleaved barcodes are excreted into the urine.
- Urine sample can be collected and barcodes analysed.
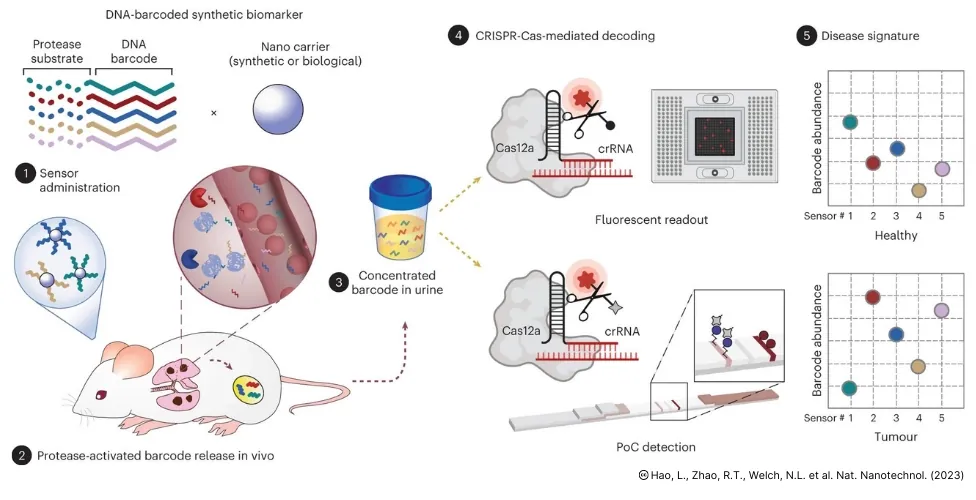
Urine samples can be analysed using a paper strip that contain different reporters that can be activated by a CRISPR enzyme called Cas12a. A particular DNA barcode in the sample is decoded together by Cas12a protein and CRISPR RNA (crRNA). They release a fluorescent signal present in the barcode, which can be detected as a colour change in the paper strip.
The researchers designed their test so that it can be performed using a strip of paper, similar to an at-home Covid test. They hope to make the test affordable and accessible to as many patients as possible.
Conducting experiments in mice, the researchers showed that they could use the sensors to detect the activity of five different enzymes that are expressed in tumours.
They were also able to scale up their system to distinguish 46 different DNA barcodes in a single sample. They achieved this using a microfluidic device to analyse the samples.
“Our goal here is to build up disease signatures and to see whether we can use these barcoded panels not only read out a disease but also to classify a disease or distinguish different cancer types,” said one of the author in a press release.
This kind of testing could be used not only for detecting cancer, but also for measuring how well a patient’s tumour responds to treatment and whether it has recurred after treatment. The researchers are now working on further developing the particles with the goal of testing them in humans. Phase 1 clinical trials have found an earlier version of these tests to be safe in patients.
